Data#
Datasets#
data()#
- data(dataset='bio_eventrelated_100hz')[source]#
NeuroKit Datasets
NeuroKit includes datasets that can be used for testing. These datasets are not downloaded automatically with the package (to avoid increasing its weight), but can be downloaded via the
nk.data()function (note that an internet connection is necessary). See the examples below.Signals: The following signals (that will return an array) are available:
ecg_1000hz: Returns a vector containing ECG signal (
sampling_rate=1000).ecg_3000hz: Returns a vector containing ECG signal (
sampling_rate=3000).rsp_1000hz: Returns a vector containing RSP signal (
sampling_rate=1000).eeg_150hz: Returns a vector containing EEG signal (
sampling_rate=150).eog_100hz: Returns a vector containing vEOG signal (
sampling_rate=100).
DataFrames: The following datasets (that will return a
pd.DataFrame) are available:iris: Convenient access to the Iris dataset in a DataFrame, exactly how it is in R.
eogs_200hz: Returns a DataFrame with
hEOG,vEOG.Single subject
Visual and horizontal electrooculagraphy
sampling_rate=200
bio_resting_5min_100hz: Returns a DataFrame with
ECG,PPG,RSP.Single subject
Resting-state of 5 min (pre-cropped, with some ECG noise towards the end)
sampling_rate=100
bio_resting_8min_100hz: Returns a DataFrame with
ECG,RSP,EDA,PhotoSensor.Single subject
Resting-state of 8 min when the photosensor is low (need to crop the data)
sampling_rate=100
bio_resting_8min_200hz: Returns a dictionary with four subjects (
S01,S02,S03,S04).Resting-state recordings
8 min (
sampling_rate=200)Each subject is DataFrame with
ECG,RSP`, ``PhotoSensor,Participant
bio_eventrelated_100hz: Returns a DataFrame with
ECG,EDA,Photosensor,RSP.Single subject
Event-related recording of a participant watching 4 images for 3 seconds (the condition order was:
["Negative", "Neutral", "Neutral", "Negative"])sampling_rate=100
eeg_1min_200hz: Returns an MNE raw object containing 1 min of EEG data (from the MNE-sample dataset).
- Parameters:
dataset (str) – The name of the dataset.
- Returns:
DataFrame – The data.
Examples
Single signals and vectors
In [1]: import neurokit2 as nk In [2]: ecg = nk.data(dataset="ecg_1000hz") In [3]: nk.signal_plot(ecg[0:10000], sampling_rate=1000)

In [4]: rsp = nk.data(dataset="rsp_1000hz") In [5]: nk.signal_plot(rsp[0:20000], sampling_rate=1000)

In [6]: eeg = nk.data("eeg_150hz") In [7]: nk.signal_plot(eeg, sampling_rate=150)

In [8]: eog = nk.data("eog_100hz") In [9]: nk.signal_plot(eog[0:2000], sampling_rate=100)

DataFrames
In [10]: data = nk.data("iris") In [11]: data.head() Out[11]: Sepal.Length Sepal.Width Petal.Length Petal.Width Species 0 5.1 3.5 1.4 0.2 setosa 1 4.9 3.0 1.4 0.2 setosa 2 4.7 3.2 1.3 0.2 setosa 3 4.6 3.1 1.5 0.2 setosa 4 5.0 3.6 1.4 0.2 setosa
In [12]: data = nk.data(dataset="eogs_200hz") In [13]: nk.signal_plot(data[0:4000], standardize=True, sampling_rate=200)

In [14]: data = nk.data(dataset="bio_resting_5min_100hz") In [15]: nk.standardize(data).plot() Out[15]: <Axes: >

In [16]: data = nk.data(dataset="bio_resting_8min_100hz") In [17]: nk.standardize(data).plot() Out[17]: <Axes: >

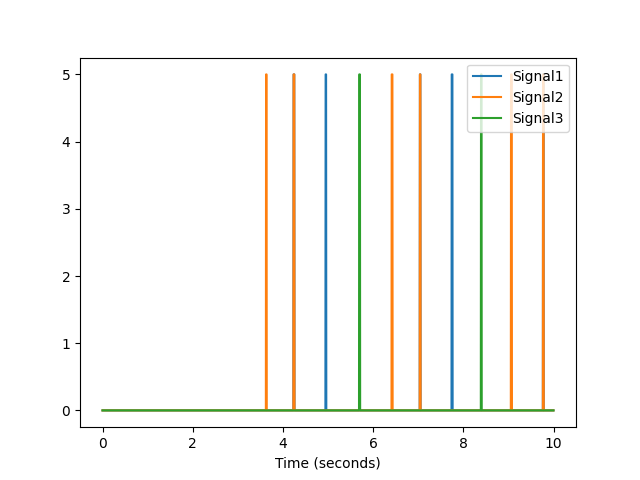
In [18]: data = nk.data("bio_resting_8min_200hz") In [19]: data.keys() Out[19]: dict_keys(['S01', 'S02', 'S03', 'S04']) In [20]: data["S01"].head() Out[20]: ECG RSP PhotoSensor Participant 0 2.394536e-19 5.010681 5.0 S01 1 1.281743e-02 5.011291 5.0 S01 2 1.129138e-02 5.010376 5.0 S01 3 7.629118e-04 5.010681 5.0 S01 4 -4.119742e-03 5.010986 5.0 S01
In [21]: data = nk.data("bio_eventrelated_100hz") In [22]: nk.standardize(data).plot() Out[22]: <Axes: >

In [23]: raw = nk.data("eeg_1min_200hz") In [24]: nk.signal_plot(raw.get_data()[0:3, 0:2000], sampling_rate=200)
I/O#
read_acqknowledge()#
- read_acqknowledge(filename, sampling_rate='max', resample_method='interpolation', impute_missing=True)[source]#
Read and format a BIOPAC’s AcqKnowledge file into a pandas’ dataframe
The function outputs both the dataframe and the sampling rate (retrieved from the AcqKnowledge file).
- Parameters:
filename (str) – Filename (with or without the extension) of a BIOPAC’s AcqKnowledge file (e.g.,
"data.acq").sampling_rate (int) – Sampling rate (in Hz, i.e., samples/second). Since an AcqKnowledge file can contain signals recorded at different rates, harmonization is necessary in order to convert it to a DataFrame. Thus, if sampling_rate is set to
max(default), will keep the maximum recorded sampling rate and upsample the channels with lower rate if necessary (using thesignal_resample()function). If the sampling rate is set to a given value, will resample the signals to the desired value. Note that the value of the sampling rate is outputted along with the data.resample_method (str) – Method of resampling (see
signal_resample()).impute_missing (bool) – Sometimes, due to connections issues, there are lapses in the recorded signal (short periods without signal). If
impute_missingisTrue, will automatically fill the signal interruptions using padding.
- Returns:
df (DataFrame) – The AcqKnowledge file as a pandas dataframe.
sampling rate (int) – The sampling rate at which the data is sampled.
See also
Example
In [1]: import neurokit2 as nk # data, sampling_rate = nk.read_acqknowledge('file.acq')
read_bitalino()#
- read_bitalino(filename)[source]#
Read an OpenSignals file (from BITalino)
Reads and loads a BITalino file into a Pandas DataFrame. The function outputs both the dataframe and the information (such as the sampling rate) retrieved from the OpenSignals file.
- Parameters:
filename (str) – Path (with or without the extension) of an OpenSignals file (e.g.,
"data.txt").- Returns:
df (DataFrame, dict) – The BITalino file as a pandas dataframe if one device was read, or a dictionary of pandas dataframes (one dataframe per device) if multiple devices are read.
info (dict) – The metadata information containing the sensors, corresponding channel names, sampling rate, and the events annotation timings if
events_annotationisTrue.
See also
Examples
In [1]: import neurokit2 as nk # data, info = nk.read_bitalino("data.txt") # sampling_rate = info["sampling_rate"]
read_video()#
- read_video(filename='video.mp4')[source]#
Reads a video file into an array
Reads a video file (e.g., .mp4) into a numpy array of shape. This function requires OpenCV to be installed via the
opencv-pythonpackage.- Parameters:
filename (str) – The path of a video file.
- Returns:
array – numpy array of shape (frame, RGB-channel, height, width).
int – Sampling rate in frames per second.
Examples
In [1]: import neurokit2 as nk # video, sampling_rate = nk.read_video("video.mp4")
write_csv()#
- write_csv(data, filename, parts=None, **kwargs)[source]#
Write data to multiple csv files
Split the data into multiple CSV files. You can then re-create them as follows:
- Parameters:
data (list) – List of dictionaries.
filename (str) – Name of the CSV file (without the extension).
parts (int) – Number of parts to split the data into.
- Returns:
None
Example
Save big file in parts
In [1]: import pandas as pd In [2]: import neurokit2 as nk # Split data into multiple files # nk.write_csv(data, 'C:/Users/.../data', parts=6)
Read the files back
# Iterate through 6-parts and concatenate the pieces # data_all = pd.concat( # [pd.read_csv(f"data_part{i}.csv") for i in range(1, 7)], # axis=0, # )
Other#
Submodule for NeuroKit.
- download_from_url(url, destination_path=None)[source]#
Download Files from URLs
Download a file from the given URL and save it to the destination path.
- Parameters:
url (str) – The URL of the file to download.
destination_path (str, Path) – The path to which the file will be downloaded. If None, the file name will be taken from the last part of the URL path and downloaded to the current working directory.
- Returns:
bool – True if the file was downloaded successfully, False otherwise.
- download_zip(url, destination_path=None, unzip=True)[source]#
Download ZIP files
Download a ZIP file from a URL and extract it to a destination directory.
- Parameters:
url (str) – The URL of the ZIP file to download.
destination_path (str, Path) – The path to which the ZIP file will be extracted. If None, the folder name will be taken from the last part of the URL path and downloaded to the current working directory.
unzip (bool) – Whether to unzip the file or not. Defaults to True.
- Returns:
bool – True if the ZIP file was downloaded successfully, False otherwise.
- read_xdf(filename, dejitter_timestamps=True, synchronize_clocks=True, handle_clock_resets=True, upsample_factor=2.0, fill_method='ffill', fill_value=0, fillmissing=None, interpolation_method='linear', timestamp_reset=True, timestamp_method='circular', mode='precise', verbose=True, show=None, show_start=None, show_duration=1.0)[source]#
Loads an XDF file, sanitizes stream data, and resamples all streams onto a common, synchronized timebase.
This function handles complex synchronization issues including clock offsets, jitter removal (selective or global), and differing sampling rates. It produces a single pandas DataFrame containing all aligned data.
Note
This function requires the pyxdf module to be installed. You can install it with
pip install pyxdf.Warning
Note that, as XDF can store streams with different sampling rates and different time stamps, the function will resample all streams to 2 times (default) the highest sampling rate (to minimize aliasing) and then interpolate based on an evenly spaced index. While this is generally safe, it may produce unexpected results, particularly if the original stream has large gaps in its time series. For more discussion, see here.
- Parameters:
filename (str) – Path to the .xdf file to load.
dejitter_timestamps (bool or list, optional) – Controls jitter removal (processing of timestamp irregularities). If
bool, passed directly to pyxdf (Trueapplies to all streams,Falseto none). Iflist, a list of stream names (str) or indices (int); dejittering is applied only to these specific streams. Note: using a list triggers a double-load of the file, increasing memory usage and loading time. Default isTrue.synchronize_clocks (bool, optional) – If True, attempts to synchronize clocks using LSL clock offset data. Passed to pyxdf.load_xdf. Default is True.
handle_clock_resets (bool, optional) – If True, handles clock resets (e.g., from hardware restarts) during recording. Passed to pyxdf.load_xdf. Default is True.
upsample_factor (float, optional) – Determines the target sampling rate for the final DataFrame. The target rate is calculated as: max(nominal_srate) * upsample_factor. Higher factors reduce aliasing but increase memory usage. Default is 2.0.
fill_method ({‘ffill’, ‘bfill’, None}, optional) – Method used to fill NaNs arising from resampling (e.g., zero-order hold). Default is ‘ffill’ (forward fill).
fill_value (float or int, optional) – Value used to fill remaining NaNs (e.g., at the start of the recording before the first sample). Default is 0.
fillmissing (float or int, optional) – DEPRECATED: This argument is deprecated and has no direct equivalent in the new implementation. It previously controlled filling of gaps larger than a threshold.
interpolation_method ({‘linear’, ‘previous’}, optional) – Method used for interpolating data onto the new timebase.
timestamp_reset (bool, optional) – If
True(default), shifts all timestamps so the recording starts at t=0.0, useful for analysis relative to the start of the specific file. IfFalse, preserves the absolute LSL timestamps (Unix epoch), useful when synchronizing this data with other files or external clocks.timestamp_method ({‘circular’, ‘anchored’}, optional) – Algorithm used to generate the new time axis.
'circular'uses a weighted circular mean to find the optimal phase alignment across all streams, minimizing global interpolation error.'anchored'aligns the grid strictly to the stream with the highest effective sampling rate. Default is'circular'.mode ({‘precise’, ‘fast’}, optional) –
'precise'uses float64 for all data, preserving precision but using more memory.'fast'uses float32, reducing memory usage by ~50% but may lose precision for very large values. Default is'precise'.verbose (bool, optional) – If True, prints progress, target sampling rates, and categorical mappings to console. Default is True.
show (list of str, optional) – A list of channel names to plot for visual quality control after resampling. If None, no plots are generated.
show_start (float, optional) – The start time (in seconds) for the visual control plot window. If None, defaults to the middle of the recording.
show_duration (float, optional) – Duration of the visual control window in seconds. Default is 1 second.
- Returns:
resampled_df (pandas.DataFrame) – A single DataFrame containing all streams resampled to the common timebase. The index is the timestamp (seconds).
See also
Examples
In [1]: import neurokit2 as nk # data, info = nk.read_xdf("data.xdf") # sampling_rate = info["sampling_rate"]